Look-alike sound-alike drugs and medication errors
Medication errors are a major cause of preventable harm to patients globally.¹ One well-recognised cause of these errors is ‘look-alike, sound-alike’ (LASA) medicines.
Look-alike medicines are visually similar with respect to packaging, shape, colour and/or size, while sound-alike medicines are similar in the phonetics of their names, doses and/or strengths.²
LASA medication errors can occur in any healthcare setting including hospitals, primary care, and care homes.¹
LASA medication errors can have serious consequences for the patient and can prove fatal.² ³
In this article, we will outline the problem of LASA errors, give some common examples, and discuss how to prevent them.
Please complete the form at the bottom of this article to request a complimentary trial of MedicinesComplete.
Definition: LASA medication errors
The NHS defines a medication error as a patient safety incident where there has been an error in the process of prescribing, preparing, dispensing, administering, monitoring, or providing advice on, medicines.⁴
In the UK, medication errors are a significant cause of patient harm, costing the NHS an estimated £98 million per year and causing, or contributing to, around 1700 deaths.⁵
There is no universally accepted definition of a LASA medication error and there are many different uses of the term ‘LASA’, so it is difficult to determine how often they occur.² ³
LASA errors do not always cause harm, as the issue may be identified before the medicine reaches the patient. This is termed a ‘near miss’. If such errors go unnoticed, however, the patient may receive the wrong medicine or dose, potentially causing serious harm.
There are wide variations in the reported prevalence of LASA errors, with a recent review article suggesting that they may occur in up to 0.0022% of all prescriptions.³
In the UK, where over 1 billion prescriptions are issued each year, this incidence extrapolates to 2.2 million such errors each year.³
What causes LASA medication errors?
LASA errors can happen at any point in the medicines use process, from prescribing, transcribing, dispensing, to administration, and monitoring.²
Many are due to confusion between medicine names. This can occur when medicines have names that appear similar when written or printed (i.e. orthographic) or sound- alike when spoken (i.e. phonetic).²
Prescribed medicines all have a generic name and, until they are out of patent, a brand name. Confusion can occur between: generic–generic names (e.g. carbamazepine-carbimazole); brand–brand names (e.g. Losec-Lasix); or brand–generic names (e.g. Malarone-mefloquine).² ³
This can happen for a variety of reasons. It can relate to how the brain works when reading. For instance in the English language, reading from left to right can create confusion when the start of a medicine name is similar. As we read by ‘chunking’ groups of letters in a word, similar endings can cause the same problem.
The term ‘look-alike’ also refers to medicines that are visually similar in shape, colour, size, or packaging. The appearance of medicines can be misleading, perhaps because very different medicines can appear to be the same or because multiple formulations and strengths of the same medicine look similar. This is especially important for ‘high-risk’ medicines, such as warfarin, opioids, and lithium.⁶
Look-alike packaging is recognised as a serious concern for health professionals.⁷
Different drugs or different strengths of the same drug can easily be mistaken for each other when they have similar packaging. Some companies adopt a standardised approach to livery, which in itself can lead to visual similarities between different products.
A recent World Health Organization report on medication safety for LASA medicines sets out the most common causes of error according to the stage of medication use (see table 1).²
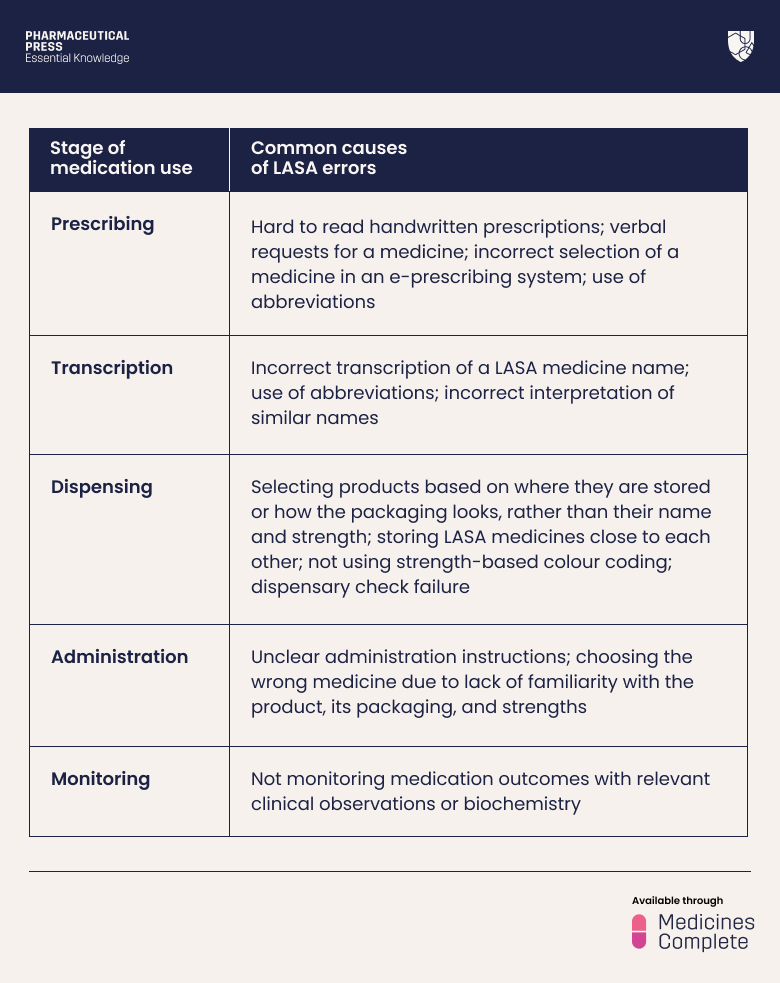
Table 1.
LASA errors and outcomes
LASA errors often result in the wrong drug being given but they can also result in overdosing, under-dosing, or inappropriate dosing.²
With respect to the clinical impact of confusing medicine pairs, the potential consequences can vary in severity, depending on factors including: the specific medicine administered, dose, therapeutic index, and route of administration. The point at which clinical teams identify and rectify a mistake will also make a difference to the level of potential harm e.g. the risk of harm will usually correlate with how long the patient takes the wrong medicine.¹ ²
There are many examples of LASA errors in published reports and case reports. For example, propranolol has been given instead of prednisolone to patients with asthma, causing bronchoconstriction and reduced blood pressure. Carbimazole, an antithyroid medicine, has been dispensed to patients prescribed carbamazepine for epilepsy, resulting in hypothyroidism and loss of seizure control.
Case reports also illustrate that these errors can have very serious outcomes. One report described mercaptopurine, rather than mercaptamine, being given to an infant with nephropathic cystinosis. The child developed the serious blood disorder, pancytopenia, but, once the mistake was spotted and rectified, made a full recovery.³
In a US case report an elderly man was given 10mg of oral hydromorphone, instead of 10mg of oral morphine in the emergency department. The error was not noticed before discharge, and the patient died from a respiratory arrest on his way home.³
Patients in extremes of age or with organ dysfunction are more vulnerable to errors and severe harm because of their physiology. The impact of LASA errors involving high-risk medicines in these patients can be serious or fatal in some instances.⁶ ⁷ ⁸
How to prevent LASA errors
Preventing LASA medication errors requires a ‘whole system’ approach. This includes naming bodies, regulators, and the pharmaceutical industry taking the potential risks into account when naming products and developing packaging.¹ ² ⁷
Regulators now take an international approach that allows LASA medicine pairs to be identified at the pre-marketing stage.²
Colour coding tablets can be useful, especially for high-risk medicines. The available strengths of warfarin tablets, for example, are colour coded by all manufacturers to differentiate them; 0.5mg (white), 1.0mg (brown), 3.0mg (blue) and 5.0mg (pink).¹
To further minimise risk, in the UK some regional prescribing policies will only allow the use of one or two strengths of warfarin tablet.
At the health system level, many steps can be taken to minimise LASA errors.² ⁷
These range from fostering a trusting patient safety culture, developing robust processes and protocols for prescribing, dispensing and medicines storage and supply. Examples include effective checking processes, ensuring that medicines with similar packaging are not stored next to each other, and discouraging handwritten prescriptions.¹ ²
The use of electronic prescribing systems enhances patient safety and reduces medication error rates but does not eliminate the possibility of LASA errors.
Educational materials and training in safe medication practice are invaluable. To help differentiate known LASA pairs, it can be useful to use “TALLman lettering” (TML). This term has been coined for writing part of a drug’s name in uppercase letters to emphasise parts of confusable names e.g. DOBUTamine and DOPamine, and cefoTAXime and cefUROXime.¹ ³
Health professionals, especially pharmacy and nursing staff, can use labels with TML or alert notices for LASA medicines, and segregate the storage of medicines with similar packaging or different strengths of high risk medicines.¹
When prescribing a medicine from a known LASA pair, it can be helpful to include both the brand and generic names to provide greater context.¹
Health professionals should also avoid using abbreviations that may lead to confusion.
At an individual level health professionals may familiarise themselves with common LASA medicines, and be mindful of the risks when prescribing, dispensing, and administering them.¹
It is also important to educate patients on the risks of LASA medicines. This can empower them to manage the medicines they are taking and to be more vigilant when taking them.¹
Why are LASA errors important?
LASA medication errors are not rare. The WHO has highlighted them as one of the most common and preventable causes of harm in healthcare.¹
With thousands of medicines available, many with similar-sounding generic or brand names, there is a high likelihood of such errors, especially as new drugs enter the market.
LASA errors are a critical patient safety concern because they can occur in every healthcare setting and at any stage of medication use. Their potential outcomes include overdosing, underdosing, or inappropriate dosing of unintended or intended medications.²
Some patients are more vulnerable to errors and severe harm, including the elderly, children and neonates and those with organ dysfunction.²
Raising awareness of the risks of LASA medication errors, good education, preventive strategies such as TML, and improved naming, packaging, labelling and prescribing practices are all essential steps in reducing errors.
Health professionals should understand the importance of reporting these errors and near-misses, so that the learning from such events can prevent them happening again. This is vital to protecting patients, improving safety culture, and ensuring the delivery of high-quality care.
Trial form
Please complete the form below to request a complimentary trial to knowledge products through MedicinesComplete.
References
- What are the most common types of medication errors? (2024) [https://www.pharmaceuticalpress.com/resources/article/what-are-the-most-common-types-of-medication-errors] Last accessed: 28 October 2025.
- World Health Organization (2023), Medication safety for look-alike, sound-alike medicines [https://iris.who.int/bitstream/handle/10665/373495/9789240058897-eng.pdf] Last accessed: 16 September 2025.
- Bryan, R., Aronson, J. K., et al. (2021). The problem of look‐alike, sound‐alike name errors: drivers and solutions. British Journal of Clinical Pharmacology, 87(2), 386-394.
- Learning from medication errors, NHS Resolution [https://resolution.nhs.uk/2023/03/30/learning-from-medication-errors] Last accessed: 29 October 2025.
- Elliott RA, Camacho E, Jankovic D, et al., Economic analysis of the prevalence and clinical and economic burden of medication error in England, BMJ Quality & Safety 2021;30:96-105.
- Coon R. High-risk medications: a guide for pharmacy professionals. Pharmaceutical Journal, 5 September 2025.
- Irish Medication Safety Network. Briefing Document on Sound-Alike Look-Alike Drugs (SALADs) in the Hospital Setting (2024). Last accessed: 28 October 2025.
- Bryan, R., Aronson, J. K., et al. (2021). A systematic literature review of LASA error interventions. British Journal of Clinical Pharmacology, 87(2), 336-351.








