 Extended Stability for Parenteral Drugs
Extended Stability for Parenteral Drugs

The only guide to safely extend the dating of parenteral drugs
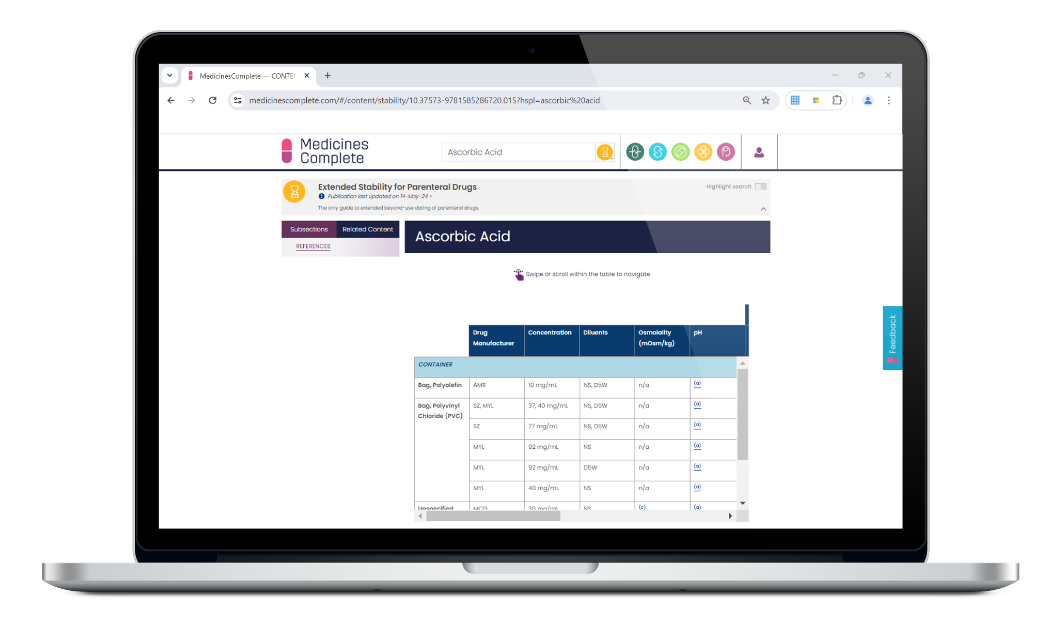
Extended Stability for Parenteral Drugs supports safe extended dating of parenteral drugs beyond the usual 24-hour limit – minimising waste and lowering medicines costs. The go-to reference for anyone preparing, administering, or compounding.
Extended Stability for Parenteral Drugs provides an extensive list of medications with temperature excursion data for intact vials. Coverage of all aspects of determining stability, including the changing elastomeric landscape and the ongoing variability in stability data. Plus, newly published data on parenteral nutrition, oncology, specialty, and COVID medications.
- 196
- Monographs
- 37
- New stability monographs
- 808
- References and communication documents
- 2
- Editors with 13 contributing writers
Safely extend beyond-use dating of parenteral medications

Quickly access extended stability information for IV solutions
Safely extend dating of parenteral medications

Minimise medicines waste from partial dosing or temperature excursions

Reduce medicines costs

Allows medicines to be prepared in bulk or ahead of time

Enable optimal patient administration

Links to ASHP Injectable Drug Information

User-friendly data in easy to digest tables
Request access today
Access Extended Stability for Parenteral Drugs through MedicinesComplete today.
Contact us now for pricing and access information.
Related resources


On-demand Webinar
20th November 2025
Safely manage patients through mental health challenges including drug discontinuation and shortages


User guide
September 2025
Palliative Care Formulary: Independent, specialist information, grounded in clinical practice

Patient case study
September 2025








