Pharmacokinetic interactions and pharmacodynamic interactionsMechanisms of drug-drug interactions (DDIs)
In this article the Stockley’s Drug Interactions expert editorial team give a brief overview of the different mechanisms by which drug interactions, including drug-drug interactions, drug-herb interactions, and drug-food interactions, occur.
These mechanisms can generally be categorised as either pharmacokinetic (PK) interactions or pharmacodynamic (PD) interactions. Many drugs that interact do so, not by a single mechanism, but often by two or more mechanisms.
Pharmacokinetic interactions
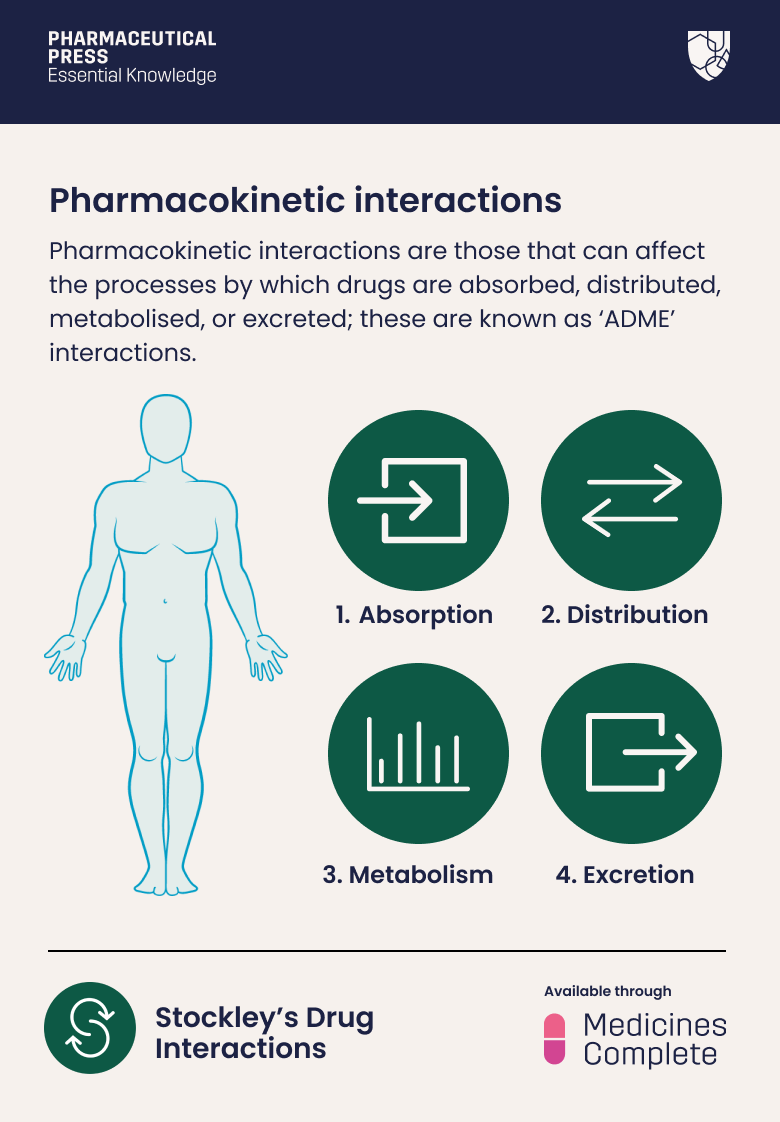
Pharmacokinetic interactions are those that can affect the processes by which drugs are absorbed, distributed, metabolised, or excreted; these are known as ‘ADME’ interactions.
Please complete the form at the bottom of this article to request a complimentary trial of MedicinesComplete.
Absorption
The absorption of most drugs taken orally takes place in the small intestine.
The rate and/or the amount absorbed can be altered by other drugs via, for example, changes in intestinal motility or gastric pH. Most of these interactions result in reduced rather than increased absorption.
A well-known absorption interaction occurs when tetracycline antibacterials chelate with a number of divalent and trivalent metallic ions, such as calcium (including when taken as milk and other dairy products) and iron, to form complexes that are both poorly absorbed and have reduced antibacterial effects.
Distribution
The distribution of drugs around the body can be affected by a number of processes.
For example, one drug can displace the protein binding of another, meaning more ‘free’ drug is available to reach the site of action and have a therapeutic effect. However, it is difficult to find an example of a clinically important interaction due to this mechanism alone.
Drugs can also induce or inhibit transporter proteins which move other drugs across membranes; this can either increase or decrease how much of the drug reaches the site of action.
The study of transporters in the pharmacokinetics of drugs is an integral part of drug development, in particular their potential role in drug interactions although evidence of their contribution to drug interactions is still emerging. Those transporters with the greatest understanding in relation to drug interactions include breast cancer resistance protein (BCRP), multidrug and toxin extrusion (MATE) proteins, organic anion transporters (OATs), organic anion-transporting polypeptides (OATPs), organic cation transporters (OCTs), and P-glycoprotein.
An example of an interaction involving a drug transporter is that between digoxin and itraconazole. Itraconazole inhibits P-glycoprotein, which transports digoxin out of kidney tubule cells into the urine. Concurrent use therefore decreases digoxin urinary clearance, and its serum concentrations and exposure are increased.
Metabolism
Although a few drugs are cleared from the body simply by being excreted unchanged in the urine, most are chemically altered within the body to less lipid-soluble compounds, which are more easily excreted by the kidneys. The greatest proportion of metabolism is carried out by the liver.
Drugs are metabolised by two major types of reaction: phase I and phase II, both of which rely on drug metabolising enzymes. The two most important pathways of drug metabolism are oxidation by cytochrome P450 (CYP) and glucuronidation by UDP-glucuronosyltransferases (UGT). A drug might be metabolised by several cytochrome P450 isoenzymes, or UGT isoforms, and a drug could be metabolised by both pathways. There is also overlap between these pathways and transporter proteins (see Distribution, above).
The most important cytochrome P450 isoenzyme is CYP3A4, followed by CYP2D6, and CYP2C9, with CYP1A2, CYP2C8, and CYP2C19 also being important. The activity of cytochrome P450 isoenzymes can be induced (increased) or inhibited (decreased) by some drugs so that their concurrent use with drugs that are known to be substrates for a particular isoenzyme might result in the exposure of the substrate being decreased or increased, respectively, necessitating dose adjustment, additional monitoring, or cessation of use.
For example, the exposure to midazolam (a substrate of CYP3A4) is markedly increased by clarithromycin (an inhibitor of CYP3A4) leading to adverse effects.
Excretion
Drug excretion mainly occurs via the kidney.
Interference by drugs with renal tubular fluid pH or with blood flow to the kidney can alter the excretion of other drugs. Interactions can also occur when two drugs compete for the same active transport system, which can delay the excretion of a particular drug.
For example, probenecid reduces the renal excretion of cephalosporins resulting in an increase in their concentrations and the possibility of toxicity. Similarly, the concentration of methotrexate can be increased by some NSAIDs with serious toxicity possible.
Pharmacodynamic drug interactions
Pharmacodynamic interactions are those where the effects of one drug are changed by the presence of another drug at its site of action resulting in additive or opposing effects.
Sometimes the drugs directly compete for particular receptors (such as beta₂ agonists and beta blockers), but often the reaction is more indirect and involves interference with physiological mechanisms.
For example, ACE-inhibitors reduce the concentration of aldosterone, which results in the retention of potassium, and this would be expected to be additive with the potassium-retaining effects of potassium sparing diuretics. Theoretically this could lead to hyperkalaemia, but usually only if other risk factors are present.

Stockley’s Drug Interactions can be accessed online via MedicinesComplete.
Where can I find more information about the mechanisms of drug interactions?
Further detailed information on the mechanisms of drug interactions can be found in Stockley’s Drug Interactions, including evidence-based classification tables for drugs which are inducers, inhibitors, and substrates of different cytochrome P450 isoenzymes and drug transporters.
Expertly authored by clinical pharmacists, Stockley’s Drug Interactions covers interactions between therapeutic drugs, proprietary medicines, herbal medicines, foodstuffs, drinks, pesticides, and drugs of abuse. It consists of concise, accurate, and clinically relevant drug interaction information based on published sources, including clinical studies, case reports, and systematic reviews.
By providing clear and practical management advice, Stockley’s Drug Interactions reliably supports health professionals decide the best course of action regarding drug interactions to deliver the best patient outcomes. Stockley’s Drug Interactions covers drugs available in the UK and Ireland, and internationally.