What is pharmacokinetics?The definition of pharmacokinetics:
“Pharmacokinetics is the study of kinetics of absorption, distribution, metabolism and excretion (ADME) of drugs and their corresponding pharmacologic, therapeutic, or toxic responses in man and animals.”¹
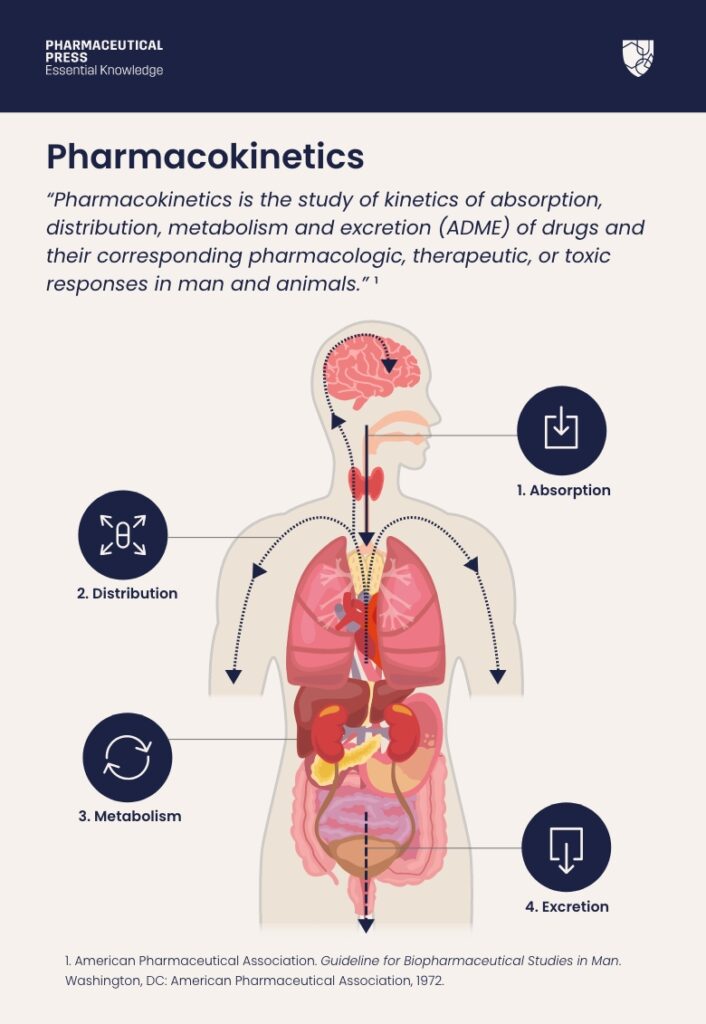
The definition of pharmacokinetics.
Applications of pharmacokinetics studies include:
● bioavailability measurements
● effects of physiological and pathological conditions on drug disposition and absorption
● dosage adjustment of drugs in disease states, if and when necessary
● correlation of pharmacological responses with administered doses
● evaluation of drug interactions
● clinical prediction: using pharmacokinetic parameters to individualise the drug dosing regimen and thus provide the most effective drug therapy.
Please note that in every case, the use must be preceded by observations.
What is Biopharmaceutics?
“Biopharmaceutics is the study of the factors influencing the bioavailability of a drug in man and animals and the use of this information to optimize pharmacological and therapeutic activity of drug products.”¹
For more article content, sign up to one of our newsletter here.
Examples of some factors include:
● chemical nature of a drug (weak acid or weak base)
● inert excipients used in the formulation of a dosage form (e.g. diluents, binding agents, disintegrating agents, colouring agents, etc.)
● method of manufacture (dry granulation and/or wet granulation)
● physicochemical properties of drugs (pKa, particle size and size distribution, partition coefficient, polymorphism, etc.).
Generally, the goal of biopharmaceutical studies is to develop a dosage form that will provide consistent bioavailability at a desirable rate. The importance of a consistent bioavailability can be very well appreciated if a drug has a narrow therapeutic range (e.g. digoxin) where small variations in blood concentrations may result in toxic or subtherapeutic concentrations.
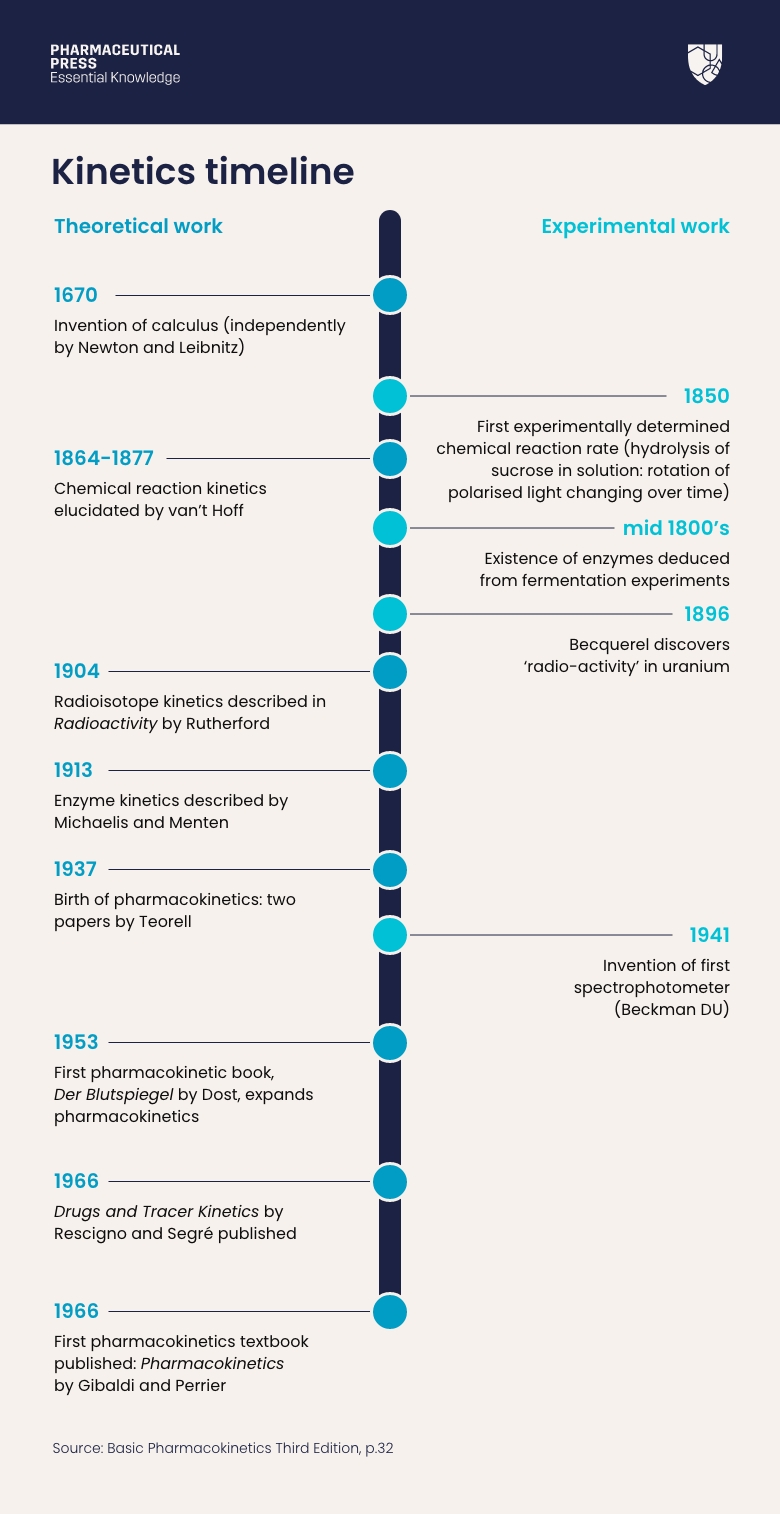
Pharmacokinetics timeline.
The content of this article has been taken from Basic Pharmacokinetics Third Edition. Order here.
Use of drugs in disease states
The use of drugs to treat or ameliorate disease goes back to the dawn of history. Since drugs are xenobiotics, that is compounds that are foreign to the body, they have the potential to cause harm rather than healing, especially when they are used inappropriately or in the wrong dose for the individual patient being treated. What, then, is the right dose? The medieval physician/ alchemist Paracelsus stated:
“Only the dose makes a thing not a poison.”
This implies: ‘the dose of a drug is enough but not too much.’
At the same time that the disciplines of medicine and pharmacy strive to use existing drugs in the most effective manner, scientific researchers are engaged in the process of discovering new drugs that are safe and effective and which are significant additions to our armamentarium for the treatment or prevention of disease. This process is increasingly time consuming, expensive, and often frustrating.
Here are two statistics about new drug approval:
● the average time for a new drug to be approved is between seven to nine years
● the cost of introducing a new drug is approximately $800 million to higher than $2 billion.
Steps involved in the drug development process include:
● The pharmacologically active molecule or drug entity must be synthesised, isolated or extracted from various possible sources (relying on the disciplines of medicinal chemistry, pharmacology, and toxicology).
● The formulation of a dosage form (i.e. tablet, capsules, suspension, etc.) of this drug must be accomplished in a manner that will deliver a recommended dose to the ‘site of action’ or a target tissue (employing the principles of physical pharmacy and pharmaceutics).
● A dosage regimen (dose and dosing interval) must be established to provide an effective concentration of a drug in the body, as determined by physiological and therapeutic needs (utilising pharmacokinetics and biopharmaceutics).
Only a successful integration of these facets will result in successful drug therapy. For example, an analgesic drug with a high therapeutic range can be of little use if it undergoes a rapid decomposition in the gastrointestinal tract and/or it fails to reach the general circulation and/or it is too irritating to be administered parenterally.
Therefore, the final goal in the drug development process is to develop an optimal dosage form to achieve the desired therapeutic goals. The optimal dosage form is defined as one that provides the maximum therapeutic effect with the least amount of drug and achieves the best results consistently.
In other words, a large number of factors play an important role in determining the activity of a drug administered through a dosage form.
A variety of disciplines are involved in understanding the events that take place during the process by which a chemical entity (substance) becomes an active drug or a therapeutic agent.
● Principles of physics, physical chemistry, and mathematics are essential in the formulation of an optimum dosage form.
● An understanding of physiology and pharmacology is essential in the process of screening for active drug and in selecting an appropriate route of administration.
● Knowledge of the principles of kinetics (rate processes), analytical chemistry and therapeutics is essential in providing an effective concentration of a drug at the ‘site of action.’ Pharmacokinetics and biopharmaceutics disciplines are the result of such a successful integration of the various disciplines mentioned above.
The first such approach was made by Teorell, when he published his paper on the distribution of drugs.² However, the major breakthrough in developing and defining this discipline has come since the early 1970s.
Further reading on Pharmacokinetics and biopharmaceutics
Pharmacokinetics and biopharmaceutics courses have been included in pharmacy curricula across the United States and in many other countries for the past several years. At present, there are a number of textbooks available for use by students and other readers. Most of these textbooks, although valuable and well written, concentrate on presenting the material in substantial mathematical depth, with much less needed emphasis on explanations which will facilitate understanding and the ability to use the pharmacokinetic equations that are introduced.
Furthermore, also evident in currently available textbooks is a paucity of adequate explanation regarding factors influencing pharmacokinetic parameters present in theses equations.
Basic Pharmacokinetics Third Edition, from which the content of this article derives, is available to order here.
About the authors of Basic Pharmacokinetics Third Edition
Sunil S. Jambhekar received his B. Pharm. Degree from L.M. College of Pharmacy of Gujarat University, India, and M.S and Ph.D. degrees in Pharmaceutics from the University of Nebraska. Prior to pursuing graduate education, Dr. Jambhekar worked for four years as a research scientist at two major pharmaceutical companies in India.
Prior to assuming his current position, Dr. Jambhekar served as an Assistant and Associate Professor of Pharmaceutics at the Massachusetts College of Pharmacy in Boston, where he was a recipient of the Trustee’s Teacher of the Year award and the Scholarly Publication award. Subsequently he was appointed Professor of Pharmaceutics at South University School of Pharmacy in Savannah, Georgia. Dr. Jambhekar has taught undergraduate and graduate courses in pharmaceutics and pharmacokinetics.
Additionally, he has directed the research of numerous graduate students, and served on the thesis advisory committees of a number of graduate students. He has authored many peer-reviewed articles and book chapters as well as scientific presentations at national and international conferences. Dr. Jambhekar has reviewed scientific books and research articles for many journals. He has been an invited external examiner for a number of doctoral candidates at colleges of pharmacy here and abroad.
Dr. Jambhekar has been a Fulbright Scholar in the lecture/research category for India as well as a Fulbright Senior Specialist and Fulbright Foundation grantee in the global/public health category. He serves as a reviewer for Fulbright Senior Specialist program. Dr. Jambhekar is an active member of several professional organizations.
Dr Philip J. Breen received his BS in Pharmacy and PhD degrees at the Massachusetts College of Pharmacy and Allied Health Sciences in Boston. For several years between undergraduate and graduate school, he was staff pharmacist and manager of a community pharmacy. For the past 35 years, Dr. Breen is a Professor at the College of Pharmacy of the University of Arkansas for Medical Sciences in Little Rock, where he teaches courses in both undergraduate and graduate pharmacokinetics. He was named Teacher of the Year at this college in 1989 and Teaching Scholar in 2009. Dr Breen has numerous national presentations and publications to his credit, as well as several grants and patents.
References
1. American Pharmaceutical Association. Guideline for Biopharmaceutical Studies in Man. Washington, DC: American Pharmaceutical Association, 1972.
2. Teorell T. Kinetics of distribution of substances administered to the body. Arch Intern Pharmacodyn 1937; 57: 205–240.2.