Pharmacodynamically inert substances
A pharmacodynamically inert substance or compound is one that has no active properties and does not produce any pharmacological effect within the body.¹
In this article, we will explain the concept in the context of pharmacodynamics (PD), and how it can relate to excipients, placebos, protein-bound drugs, and prodrugs.
Please complete the form at the bottom of this article to request a complimentary trial of MedicinesComplete.
What does pharmacodynamically inert mean?
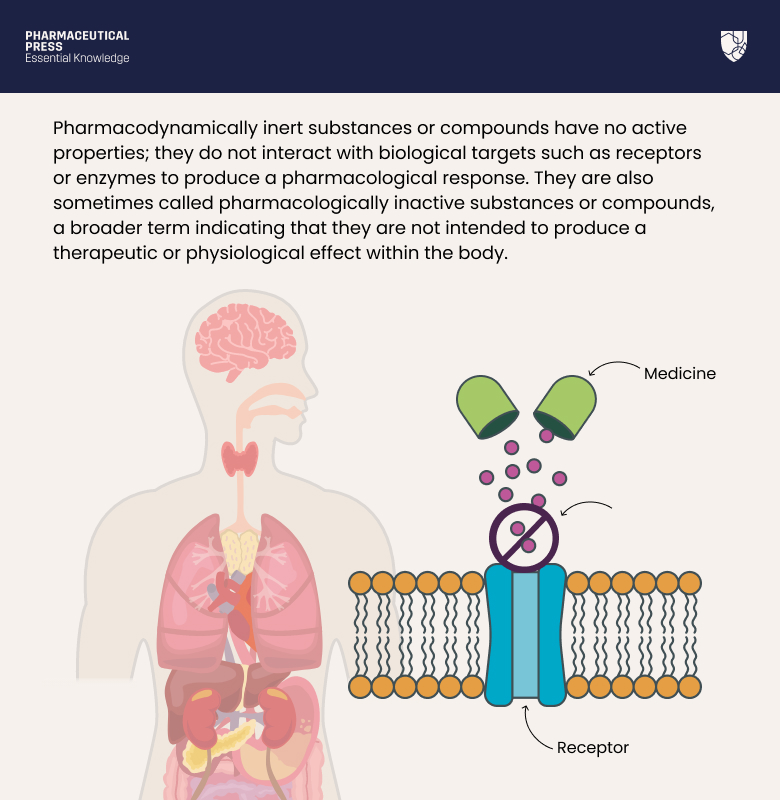
Pharmacodynamically inert substances or compounds have no active properties; they do not interact with biological targets such as receptors or enzymes to produce a pharmacological response. They are also sometimes called pharmacologically inactive substances or compounds, a broader term indicating that they are not intended to produce a therapeutic or physiological effect within the body.
Pharmacological inactivity is an important concept in the context of PD, which is the measure of the effect of a drug on the body, such as its molecular, biochemical, and physiological effects or actions.²
Pharmacodynamically inert substances and compounds play an important role in many aspects of medicine design and development. These include:
- Excipients
The International Pharmaceutical Excipient Council defines excipients as “any substance, other than an active drug or pro-drug, that is included in the manufacturing process or is contained in finished pharmaceutical dosage forms.”³ Almost all drug dosage forms include some kind of excipient.⁴ They can be used as binders, diluents, lubricants, or emulsifying or sweetening agents, for example.³
For more information, what is an excipient?
Traditionally, excipients were biologically inert substances of natural origin, such as corn, wheat, sugar, and minerals. However, more complex, and sometimes active, excipients have become available in recent years.³
- Placebos
A placebo is a pharmacodynamically inert substance that looks the same as, and is given in the same way as, an active drug.⁵
Their primary use is in placebo-controlled clinical trials, which are considered the gold standard for assessing the efficacy of new treatments. These studies allow researchers to evaluate the effectiveness of an active drug compared to that of an inactive substance.⁶
There is also evidence to suggest that some health professionals use inactive substances in clinical practice to elicit the so-called “placebo effect”.⁷ ⁸
This relates to a treatment in which the benefits derive from positive patient expectations, rather than the physiological mechanism of the treatment itself.
Sugar pills and saline solutions are examples of pharmacodynamically inert placebos.
- Protein-bound drugs
Once a drug enters the bloodstream, a proportion may bind to plasma proteins, while the remaining will exist as “free drug”. The binding of drugs to the plasma proteins is reversible, with an equilibrium established between the proportions of drug that are bound and unbound. Only the free, unbound drug can cross into tissues and interact with receptors to produce a pharmacological effect. The protein-bound drug is unable to pass into the tissues, rendering it pharmacodynamically inactive, or inert. Over time, as the free drug is metabolised and eliminated, more bound drug is released.⁹
The extent of protein binding varies widely among drugs; for example, warfarin is approximately 99% protein bound, phenytoin around 90%, while lithium exhibits no protein binding.
Albumin is one of the most important proteins in the blood. When albumin levels are low, there is less protein available for drugs to bind to resulting in a higher proportion of unbound (free) drug. This can alter how some drugs are distributed and cleared from the body, sometimes requiring dose adjustment.
- Prodrugs
A prodrug is a compound that is pharmacodynamically inert until it is metabolised to the active drug. A well-known example is codeine, which is converted by the liver enzyme CYP2D6 into morphine.¹⁰ Prodrugs are often designed to enhance drug delivery, improve absorption or targeting, and minimise adverse effects by delaying activity until the drug reaches the intended site of action.¹⁰
While pharmacodynamically inert substances have no active effect on the body, they can play key roles in drug formulation, clinical trials, and drug delivery.
Trial form
Please complete the form below to request a complimentary trial to knowledge products through MedicinesComplete.
References
1. Oxford Advanced Learner’s Dictionary. (n.d.). Inert. Available at: https://www.oxfordlearnersdictionaries.com/definition/english/inert?q=inert Last accessed: 24 April 2025.
2. Marino M, Jamal Z, et al. (2023). Pharmacodynamics. Available at: https://www.ncbi.nlm.nih.gov/books/NBK507791/ Last accessed: 24 April 2025.
3. Kar M, Chourasiya Y, et al. (2019) Current developments in excipient science: implication of quantitative selection of each excipient in product development. In Basic Fundamentals of Drug Delivery (pp. 29-83). Academic Press.
4. Kerlin RL, Li X. (2013) Pathology in non-clinical drug safety assessment. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology (pp. 725-750). Academic Press.
5. National Cancer Institute. (n.d.). Placebo. Available at: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/placebo Last accessed: 24 April 2025.
6. Gupta, U., Verma, M. (2013). Placebo in clinical trials. Perspectives in Clinical Research, 4(1), 49-52.
7. Tilburt, J. C., Emanuel, E. J., et al. (2008). Prescribing “placebo treatments”: Results of national survey of US internists and rheumatologists. BMJ,337.
8. Howick, J., Bishop, F. L., et al. (2013). Placebo use in the United Kingdom: results from a national survey of primary care practitioners. PloS One, 8(3), e58247.
9. Ernstmeyer K., Christman E., et al. (2023) Pharmacokinetics & Pharmacodynamics. In Nursing Pharmacology (Internet).
10. Choudhary, D., Goykar, H., et al. (2020). Prodrug design for improving the biopharmaceutical properties of therapeutic drugs. In The Future of Pharmaceutical Product Development and Research (pp. 179-226). Academic Press.







