Global knowledge to help grow your drug development pipeline

Cancer treatment continues to be an area of focus for drug pipeline development in the pharmaceutical sector, with a particular focus on the immunological approaches to the treatment of malignant disease. Chimeric antigen receptor (CAR) T-cell therapy is one of the most recent innovative immunotherapies and is rapidly evolving.
The antineoplastics monographs in Martindale: The Complete Drug Reference cover established cytotoxic drugs and newer agents, including PI3K inhibitors and PARP inhibitors alongside the latest CAR T-cell therapies.
Antineoplastics
Antineoplastic drugs are used as the primary treatment in responsive malignant neoplasms. They may be given when surgery or radiotherapy is not possible or has proven ineffective or combined in different regimens with surgery or radiotherapy.
Over the years, conventional cytotoxic approaches for neoplastic diseases have been developed. Due to the heterogeneity of cancer cells, the effectiveness of cytotoxic approaches is limited. This has prompted the search for more effective treatments, with considerable interest in immunological approaches.
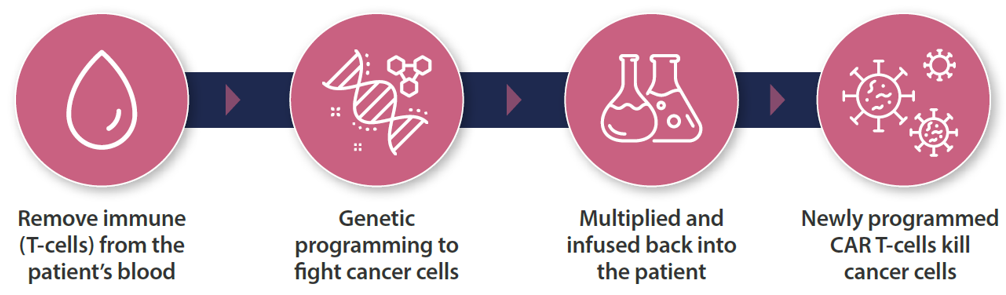
CAR T-cell therapy involves genetic modification of the patient’s T-cells to express a CAR specific for a tumour antigen, followed by ex vivo cell expansion and re-infusion back to the patient.
How does CAR T-cell therapy work?
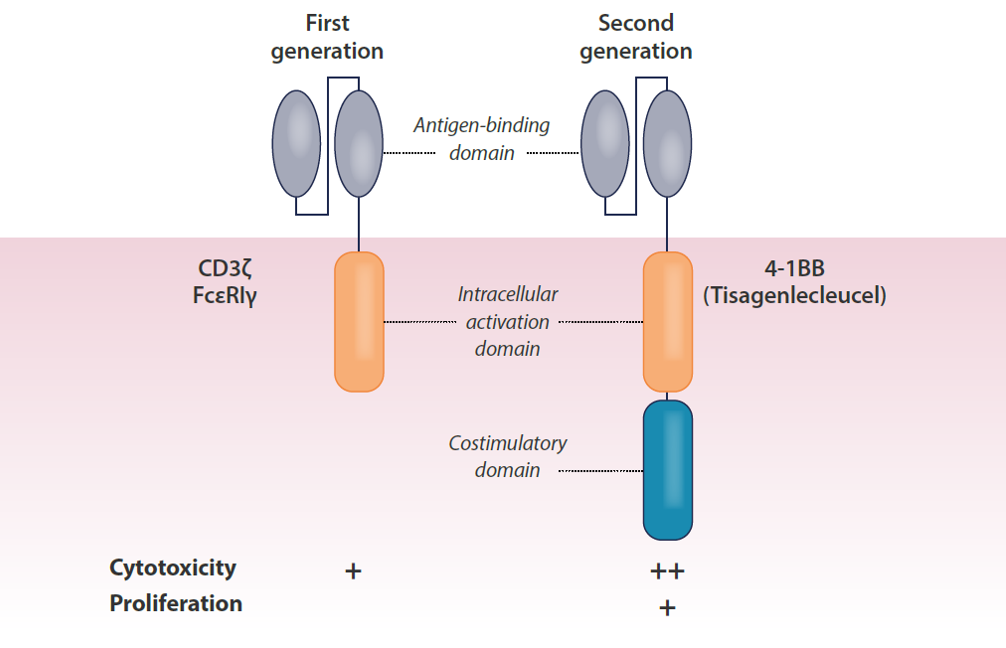
CARs have revolutionised the treatment of relapsed and refractory haematological malignancies. Through targeting of the CD19 antigen on B-cells, durable remissions have been achieved using axicabtagene ciloleucel in patients with B-cell non-Hodgkin lymphoma and tisagenlecleucel in acutely.
First and second-generation CARs
Martindale: The Complete Drug Reference informs each stage of the development process, from compound analysis through formulation development, regulatory compliance and quality assurance. The trusted information includes over 7,600 drug monographs, over 200,000 preparations from 43 countries and regions and over 25,000 international manufacturers and distributors.