Sodium nitrate and sodium nitrite poisoningAnd other nitrogen-based poisons. What are they and why are they challenging for the analytical toxicologist?
Nitrogen-based poisons like sodium nitrate/nitrite or sodium azide are increasingly encountered in suicide cases following upwards trends in internet-purchased ‘suicide kits’ [Bugelli 2022]; recreational abuse of others like nitrous oxide is also on the rise, prompting the UK government to legislate nitrous oxide as a Class C drug in 2023 [Home Office 2023].
These substances present an analytical challenge to the toxicologist due to their elemental and, in the case of nitrous oxide, volatile nature, which restricts the options available for quantitative analysis. Difficulties differentiating between endogenous and exogenous origin of nitrite and nitrate ions can also mean interpreting quantified results can be challenging.
This paper aims to explore sodium nitrate/nitrite, sodium azide, and nitrous oxide from a toxicological perspective, and address these analytical and interpretive difficulties.
Please complete the form at the bottom of this article to request a complimentary trial of MedicinesComplete.
What are sodium nitrate and sodium nitrite?
Nitrate refers to NO3 and nitrite refers to NO2, both of which can combine with salts to form compounds used in a variety of industries. For instance, ammonium nitrate (NH4NO3) is a commonly used fertilizer [Bugelli 2022], while sodium nitrate (NaNO3) and sodium nitrite (NaNO2) are preservatives added to cured meats like tinned tuna or salami [Karwowska 2020]. Sodium nitrate can also be used in the production of fertilizers and explosives, and sodium nitrite is also used clinically in the treatment of cyanide poisoning.
What are the dangers associated with sodium nitrate/nitrite poisoning?
Although sodium nitrate and nitrite are regulated as food additives in purchased food, they may be toxic above certain levels, and unregulated online access has contributed to an increase in accidental poisonings for people “home curing” food [Padovano 2022]. Furthermore, easy online access to “suicide kits” [Bugelli 2022] containing sodium nitrate/nitrite (and sometimes also an anti-emetic to prevent vomiting and aid its retention) has been increasingly attributed to deaths. When ingested, nitrite ions (NO2–) react with haemoglobin (Hb) to produce methaemoglobin (MetHb), resulting in systemic hypoxia. Nitrate ions (NO3–) will also convert to nitrite ions (NO2–) to some extent. In both cases, nitric oxide can also be produced, resulting in vasodilation that can adversely affect blood pressure.
What is sodium azide?
Sodium azide (NaN3), when mixed with water or an acid, rapidly hydrolyses to form an explosive gas; it is used commercially as a propellant in vehicular airbags, to facilitate reactions in chemical laboratories, and as a bactericide in biological laboratories [Tat 2021].
What are the dangers associated with sodium azide poisoning?
Sodium azide is a highly toxic compound. Its hazardous nature means commercial use is internationally regulated; in the UK this is through the Health and Safety Executives’ Control of Substances Hazardous to Health (COSHH). However, online availability has resulted in sodium azide increasingly becoming established as a method of suicide [Ingrid 2022]; lacking a targeted antidote, medical treatment is challenging [Groeneveld 2022].
What is nitrous oxide?
Nitrous oxide (N2O) is used medically as an analgesic/anaesthetic, and in the catering industry as an aerosol propellant in whipped cream cannisters. However, it has found more widespread infamy as a recreational drug (colloquially called “laughing gas” or “hippy crack”) due to the short-lived euphoric effect produced when inhaled; cannisters are readily available for purchase on social media platforms targeting this mode of use [EMCDDA 2022]. In 2023, in an attempt to supress recreational use, the UK government placed nitrous oxide into Class C of the Misuse of Drugs Act 1971 [Home Office 2023].
What are the dangers associated with nitrous oxide abuse?
The dangers associated with nitrous oxide abuse include a risk of asphyxia/hypoxia with prolonged use or if the method of inhalation is plastic bag/face mask. Disorienting effects also increase the risk of accidents, such as falls and road traffic incidents [EMCDDA 2022]. With chronic use, direct toxicity can occur due to nerve damage via inactivation of vitamin B12 [Xiang 2021].
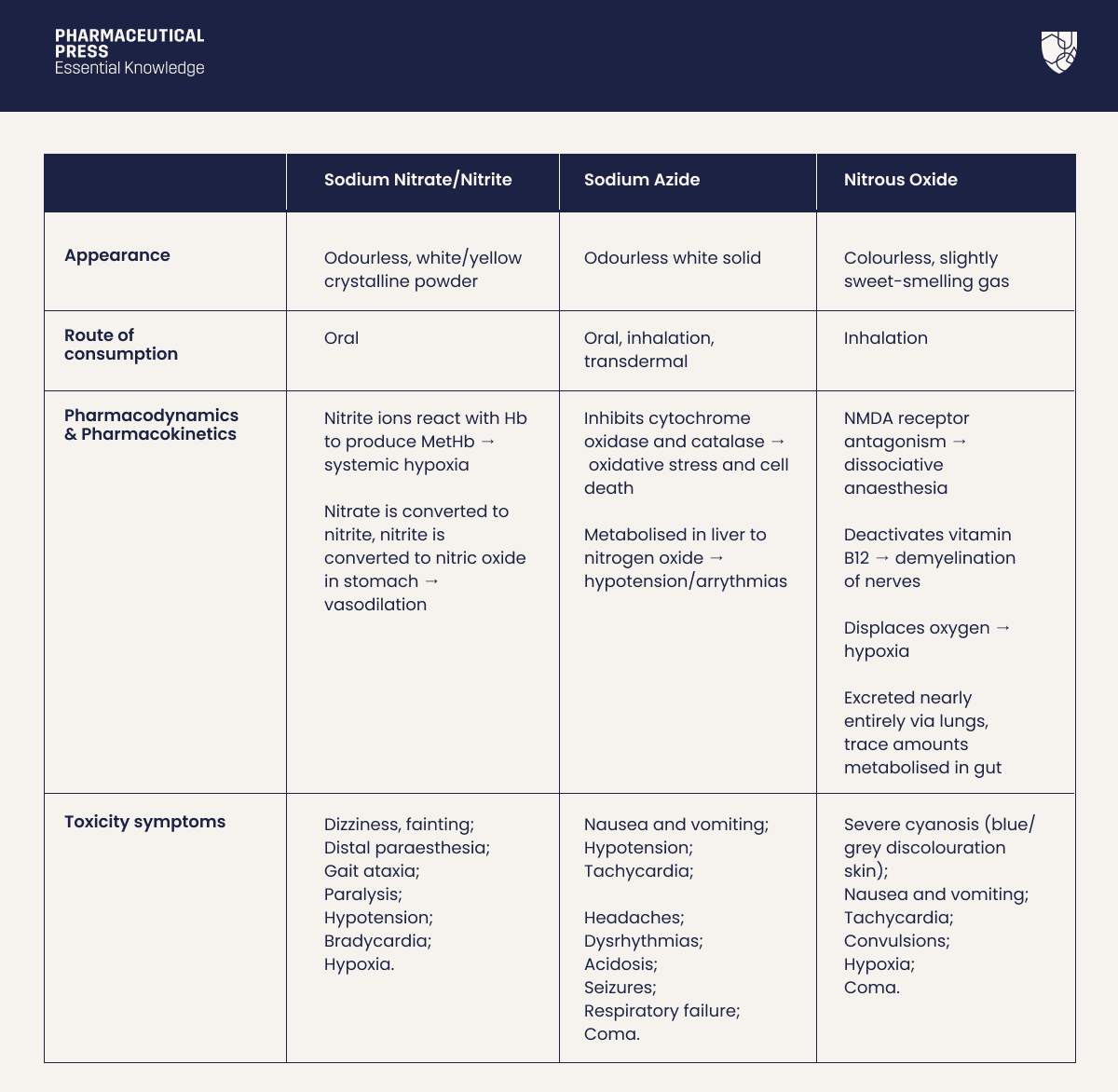
Table I: An overview of the physical, pharmacological, and toxicological properties of sodium nitrate/nitrite, sodium azide, and nitrous oxide.
Challenges and Guidance
How can nitrogen-based poisons be analytically confirmed and quantified?
Nitrogen-based compounds provide unique analytical challenges, as their simple elemental nature prohibits them from traditional toxicological detection methods. In forensic cases, there may also be a lack of awareness of scene evidence indicators to direct the analytical approach; for instance, sodium azide becomes clear and odourless when added to water and can easily be mistaken for drinking water [Tat 2021]. These compounds are not routinely tested for and consequently their presence may be overlooked in analytical investigations; evidence at the scene and eyewitness testimony are key to prompt the completion of appropriate specific screenings.
Sodium nitrate/nitrite and azide are not used recreationally and are most often implicated in suicide scenarios [Tat 2021; Hikin 2023]. Conversely, volatile substances such as nitrous oxide have a higher proportion of accidental/misadventure deaths following deliberate use [ONS 2022]. As all three compounds are readily available for purchase online, internet browsing history as part of any investigation could provide information as to their possible use and direct the need for analysis.
Sodium Nitrate/Nitrite
- Scene evidence may include nitrite/nitrate labelled bags/containers, ‘suicide kits’, white powders, nearby food/drinks which may contain the substance
- Analytical focus is on nitrite/nitrate ions
- Various techniques can be used including non-chromatographic methods (spectrophotometry, colorimetry or chemiluminescence) and chromatographic methods (ion chromatography, capillary electrophoresis, GC-ECD-MS or HPLC-UV/DAD)
- For chemiluminescence detection, conversion of nitrite/nitrate ions to NO is required, by addition of reducing agent (e.g. glacial acetic acid). The NO is subsequently released by potassium iodide to react with ozone (O3) creating an excited state, which upon spontaneously returning to a ground state releases a photon for detection [Hikin 2023]
- For chromatographic methods (e.g. HPLC-UV/DAD), the Griess reaction is utilised; nitrite ions are reacted under acidic conditions with sulfanilic acid and N-(1-naphthyl)ethylenediamine to produce a stable ‘azo’ compound with a purple colour. Absorbance at 540 or 548nm is monitored [Zhang 2023; Liu 2023]
- Biomarkers of use: MetHb, which can be measured by spectrophotometry.
Sodium Azide
- Scene evidence may include a container or product labelled sodium azide, which in almost all suicide cases was found at scene [Ingrid 2022]
- HPLC or ion chromatography is often used and may require use of specific chemical reactions to aid specificity [Kage 2000]
- For instance, extractive alkylation utilising the reaction of pentafluorobenzyl chloride with azide ions (N3–) forming a pentafluorobenzyl complex allows subsequent analysis via GC-MS and GC-EMD for detection in blood and urine [Kage 2000]
- Ion chromatography can be applied if analysis of a blood sample is preceded by a microdiffusion step in which hydrazoic acid gas (HN3), produced by acidifying the azide anion, is trapped in an alkaline solution before adding ferric chloride. This method can measure blood concentrations down to 100 ng/ml [Kruszyna 1998]
- HPLC-DAD analysis has also been described with the use of pre-column 3,5-dinitrobenzoyl chloride derivatisation and direct analysis of fluids [Lambert 1995]
- For analysis via GC-NPD, azide in blood or plasma can be derivatized in situ with propionic anhydride to yield gaseous propionyl azide, which then undergoes thermal rearrangement to form ethyl isocyanate [Meatherall 2009].
Nitrous Oxide
- Scene evidence may include the presence of cannisters and balloons; nitrous oxide has also been implicated increasingly in road traffic incidents, however there is currently no roadside or equivalent presumptive detection [Vinckenbosch 2024]
- Rapid loss from samples is possible, and it is recommended to keep samples sealed or to undertake time-sensitive analysis
- Headspace GCMS with a carbon monoxide (CO) internal standard has been used [Giuliani 2015]
- Ideal gas law can be used to determine calibration curve
- Tissue samples can confirm hypoxic damage [Cipolloni 2022]
- Biomarkers of chronic use: vitamin B12 deficiency [Marie 2022].
Scientists globally rely on Clarke’s Analysis of Drugs and Poisons to support confident analysis and interpretation of toxicology data.
Interpretation guidance
Combining circumstantial and qualitative evidence with quantified results is essential for drawing interpretative conclusions for all of these compounds.
Sodium Nitrite/Nitrate
Despite increased amounts of MetHb being produced after nitrate/nitrite ingestion, levels are not always elevated in poisoning cases and thus it cannot be relied upon as a biomarker in isolation [Hikin 2023]. However, signs of hypoxia and cyanosis (ashen/dusky grey skin) at post-mortem/autopsy are often reported in cases [Hikin 2023].
As nitrate/nitrite ions are found endogenously and ingested via a regular diet, it is important to be able to differentiate poisoning cases; reported fatalities are typically associated with concentrations that far exceed those from dietary intake. Naturally occurring concentrations of nitrates and nitrites in blood range from 0.1-0.4µmol/L and 20-40µmol/L, respectively. In a case series of 20 fatalities, the mean post-mortem blood concentration for nitrates was 6,752µmol/L (range 1,342-12,735µmol/L) and nitrite 1,873µmol/L (range 19-3,662µmol/L) [Hikin 2023]. However, as nitrite ions rapidly convert to nitrate ions naturally and may not be detected, nitrate levels have been described as more crucial in determining intoxication [Bugelli 2022]. Nevertheless, due to interchangeability and concentrations being far higher in fatalities, combined nitrite/nitrate ion concentrations in post-mortem blood greater than 1000 µmol/L (>1 mmol/L) are usually found [Elliott 2024].
Between 2018-2023 there were 25 deaths identified by the National Programme on Substance Use Mortalities (NPSUM) in the UK [NPSUM 2024], although some deaths may not have been reported due to sodium nitrites/nitrates not designated as psychoactive substances. An additional study for a two-year period (2020-2022) identified 20 cases of fatal poisonings in the UK, with the report confirming nitrates/nitrates as an emerging method of suicide [Hikin 2023]. There are similar cases reported worldwide.
Sodium nitrate/nitrite are often encountered as part of a “suicide kit” containing anti-emetic drugs, such as domperidone and metoclopramide, taken by the decedent to prevent vomit-induced evacuation of the nitrite/nitrate. Consequently, these have been co-detected in toxicological findings [Bugelli 2022; Hikin 2023].
Sodium Azide
Sodium azide toxicity presents similarly in nature to cyanide poisoning [Van der Heijden 2023], although cyanide antidotes are ineffective against sodium azide poisoning [Meatherall 2009]. Elevated levels of cyanide have been detected after sodium azide ingestion, although the metabolic production pathway is unclear [Lambert 1995; Bruin 2021]. Ensuring analysis of sodium azide is free of interference from cyanide is recommended [Meatherall 2009].
The failure to sometimes detect sodium azide in post-mortem fluids may be accounted for by its short half-life, therefore it may be prudent to confirm how long the decedent survived after ingestion [Meatherall 2009]. In one reported case, significant amounts of sodium azide were present in gastric contents, but not detected in post-mortem blood. In light of this, checking multiple tissue samples and gastric content is advisable [Le Blanc-Louvry 2012].
Between 2000-2020, 156 poisoning cases were identified, with 22 of these ending in fatalities and completed suicides accounting for 18 of these [Tat 2021]. In 11 suicide cases the range of sodium azide blood concentrations was 13-26 mg/L with a mean of 17 mg/L [Ingrid 2022].
Nitrous Oxide
Due to the analytical challenges outlined above, nitrous oxide may not be detected even when tested for. Adding further complication, decreases in vitamin B12 levels are not consistently found and may not apply to acute use, making this is an unreliable biomarker. However, increases in homocysteine levels appear more systematic, and could be indicative of recent nitrous use [Grzych 2023]. This potential lack of confirmed quantitative data highlights the importance of circumstantial evidence; some decedents are found surrounded by distinctive cannisters or ‘whippits’, and other suggestive paraphernalia such as face masks connected to cannisters or balloons that are used to inhale the gas. Where nitrous oxide was detected, a concentration range of 3.2-45mg/L has been found in the post-mortem blood of fatal cases [Elliott 2024]. However, the concentration is not deemed essential, and it is considered more important to confirm detection as there should be no measurable nitrous oxide present in blood normally.
Between 2001-2020 nitrous oxide was mentioned on a death certificate 56 times in the UK [ONS 2022]. The rise in nitrous oxide-related road traffic fatalities does provide its own unique interpretive challenge, as there is no clear guidance on the impact of nitrous oxide use on driving abilities [Vinckenbosch 2024]. There is also case literature worldwide.
Trial form
Please complete the form below to request a complimentary trial to knowledge products through MedicinesComplete.
References
Bugelli V et al. Four cases of sodium nitrite suicidal ingestion: A new trend and a relevant forensic pathology and toxicology challenge. Legal Medicine 2022; 59:102146.
Karwowska M, Kononiuk A. Nitrates/nitrites in food-risk for nitrosative stress and benefits. Antioxidants (Basel) 2020; 9(3): 241.
Tat J et al. Sodium azide poisoning: A narrative review. Clin Toxicol (Phila) 2021; 59(8): 683-697.
Ingrid B et al. A case series of suicides by sodium azide ingestion. Toxicologie Analytique et Clinique 2022; 34(3): S45-S46.
Groeneveld N et al. Potential therapies for sodium azide intoxication: A case report and review of the literature. Acute Med 2022; 21(2): 86-95.
EMCDDA. Rapid communication: Recreational use of nitrous oxide — A growing concern for Europe, November 2022. https://www.euda.europa.eu/publications/rapid-communication/recreational-use-nitrous-oxide-growing-concern-europe_en (accessed 04 June 2025).
Xiang Y et al. Recreational nitrous oxide abuse: Prevalence, neurotoxicity, and treatment. Neurotox Res 2021; 39(3): 975-985.
Hikin LJ et al. Sodium nitrite poisoning: A series of 20 fatalities in which post-mortem blood nitrite and nitrate concentrations are reported. Forensic Sci Int 2023; 345: 111610.
Kage S et al. Determination of azide in blood and urine by gas chromatography-mass spectrometry. J Anal Toxicol 2000; 24(6): 429-432.
Kruszyna R et al. Determining sodium azide concentration in blood by ion chromatography. J Forensic Sci 1998; 43(1): 200-202.
Lambert WE et al. Application of high-performance liquid chromatography to a fatality involving azide. J Anal Toxicol 1995; 19(4): 261-264.
Meatherall R, Palatnick W. Convenient headspace gas chromatographic determination of azide in blood and plasma. J Anal Toxicol 2009; 33(8): 525-531.
Vinckenbosch FRJ et al. The prevalence, risks, and detection of driving under the influence of nitrous oxide. WIREs Forensic Science 2024; 6(2): e1508.
Giuliani N et al. Validation of an analytical method for nitrous oxide (N2O) laughing gas by headspace gas chromatography coupled to mass spectrometry (HS-GC–MS): Forensic application to a lethal intoxication. J Chromatogr B Analyt Technol Biomed Life Sci 2015; 983-984: 90-93.
Cipolloni L, Simone SD. Nitrous oxide intoxication: Systematic literature review and proposal of new diagnostic possibilities. Egypt J Forensic Sci 2022; 12.
Marie JCC et al. Nitrous oxide abuse in the emergency practice, and review of toxicity mechanisms and potential markers. Food Chem Toxicol 2022; 162: 112894.
van der Heijden LT et al. Internet-purchased sodium azide used in a fatal suicide attempt: A case report and review of the literature. Toxics 2023; 11(7): 608.
Bruin MAC et al. Toxicological analysis of azide and cyanide for azide intoxications using gas chromatography. Basic Clin Pharmacol Toxicol 2021; 128(3): 534-541.
Le Blanc-Louvry I et al. Suicidal sodium azide intoxication: An analytical challenge based on a rare case. Forensic Sci Int 2012; 221(1-3): e17-20.
Grzych G et al. Comparison of biomarker for diagnosis of nitrous oxide abuse: challenge of cobalamin metabolic parameters, a retrospective study. J Neurol 2023; 270(4): 2237-2245.
Zhang M et al. Presumptive identification of nitrite by Griess reagent test strips—Case reports of fatal poisoning with sodium nitrite. J Anal Toxicol 2023; 47: 746–749
Liu, G et al. Advancements in preprocessing and analysis of nitrite and nitrate since 2010 in biological samples: A review. Molecules 2023; 28: 7122.
Elliott, SP. Analytical and interpretative challenges for emerging nitrogen-based poisons. Analytica conference 2024, Munich.
Copeland C, Kalk N. The National Programme on Substance Use Mortality (NPSUM). https://www.kcl.ac.uk/research/the-national-programme-on-substance-use-mortality (accessed 03 June 2024).
Padovano M et al. Sodium nitrite intoxication and death: Summarizing evidence to facilitate diagnosis. Int J Environ Res Public Health. 2022;19(21):13996.
Home Office. Circular 006/2023: Control of nitrous oxide under the Misuse of Drugs Act 1971. November 2023. https://www.gov.uk/government/publications/circular-0062023-control-of-nitrous-oxide-under-the-misuse-of-drugs-act-1971 (accessed 04 June 2025).
Office for National Statistics. Deaths related to volatile substances, helium and nitrogen in England and Wales: 2001 to 2020 registrations. February 2022. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/articles/deathsrelatedtovolatilesubstancesheliumandnitrogeninenglandandwales/2001to2020registrations (accessed 03 June 2025).